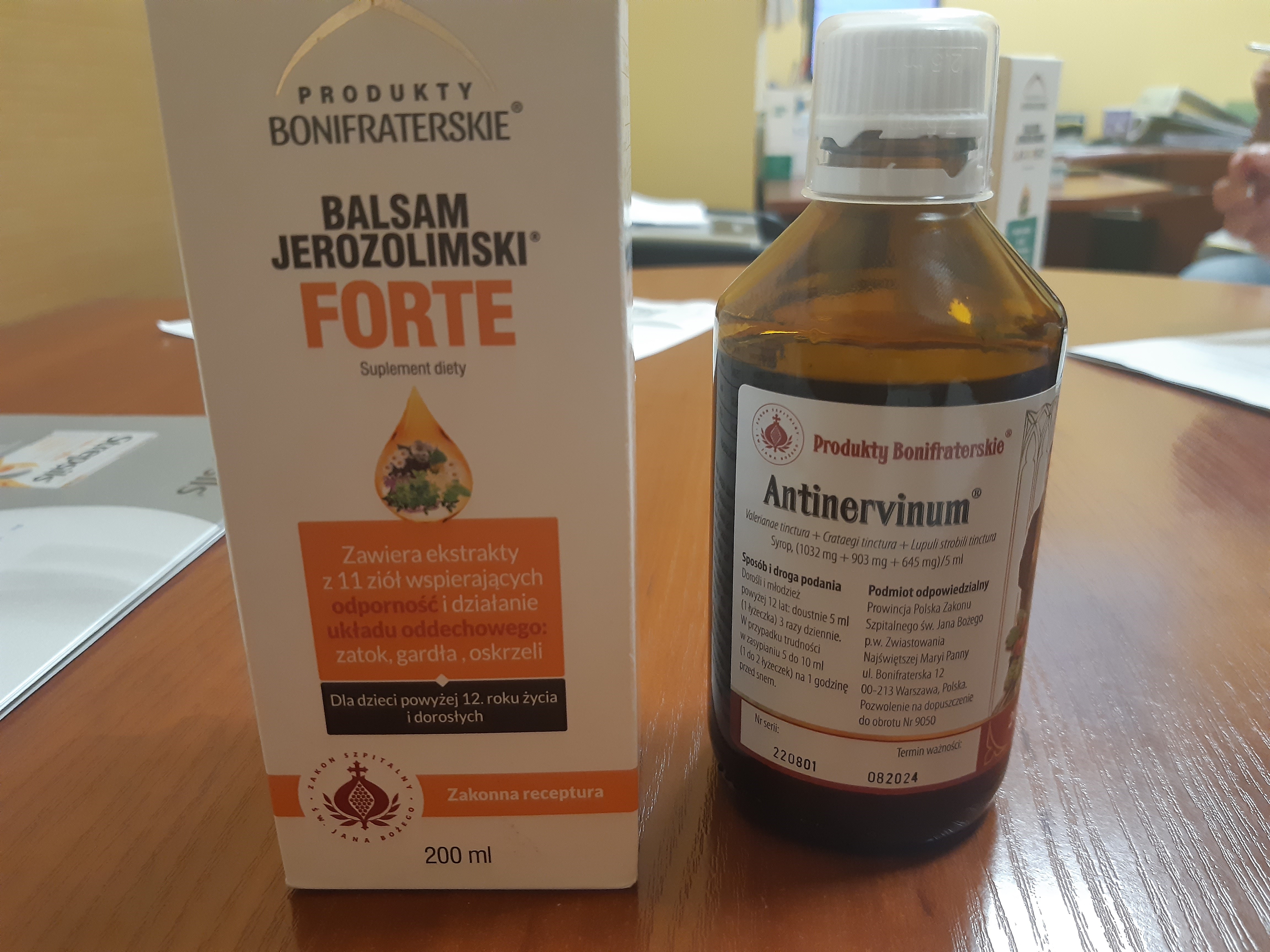
Jerusalem Balm FORTE dietary supplement 200 ml
Bottles containing a liquid labeled as the medicinal product Antinervinum were found inside the supplement packaging, indicating inconsistent labeling and content. Potential effects: unintended drug intake, adverse reactions, poisoning, complications.
Download the app and stay informed
Get instant notifications about recalled products and protect your family.